- Article
Bridging gaps in neurology trials: How community-based research models expand access
As momentum continues to grow in neurology research, how can community-based research models help overcome recruitment and retention challenges in complex areas like Alzheimer's?
According to data published in a landmark study tracking the global burden of diseases, more than 3 billion people worldwide are living with a neurological condition – disorders that impact the brain, spinal cord, and nerves. These diseases are now the leading cause of ill health and disability worldwide, ranging from sudden onset conditions such as stroke and spinal cord injuries to more progressive, degenerative diseases like Alzheimer’s and Parkinson’s. Neurodegenerative diseases are estimated to affect 50 million people worldwide, yet their root causes and progression remain poorly understood, limiting the development of targeted therapies.
Given the disproportionate impact of neurological conditions on older adults, the global burden of these diseases is expected to rise significantly as the population continues to age. In response to the urgent need for more disease-modifying therapies, drug development efforts in neurology have continued to expand. One clear indicator of this growth is the 76% increase in new clinical trials for neurological indications between 2015 and 2024 – 22% higher than the average growth across all clinical trial areas. 2023 marked a particularly strong year for neurology research, with more than 2,000 new studies initiated worldwide.
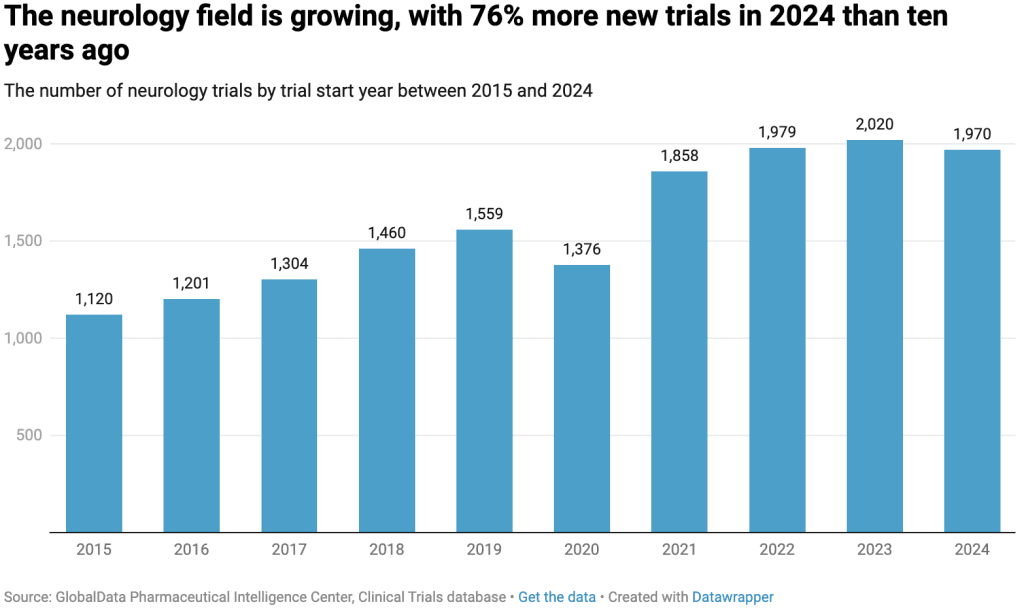
Further analysis confirms that the Central Nervous System therapy area, an umbrella term which encompasses neurological and psychiatric conditions, is now a leading focus of the pharmaceutical industry. By comparing the number of planned and ongoing clinical trials across all therapy areas in April 2025, Central Nervous System emerged second highest, after oncology.
What are the challenges in neurodegenerative disease trials?
As clinical trial activity in neurology increases, several recurring challenges have become apparent, including the use of subjective endpoints to assess cognition, memory, and behavior. Furthermore, poor patient recruitment, lower-than-average protocol compliance, and extended study durations contribute to the substantial costs associated with running these complex and extended trials. Analysis by GlobalData shows that Phase III neurology trials are more likely to experience slower enrollment compared to Phase III trials in other therapeutic areas. The table below shows the average enrollment efficiency and enrollment period duration for Phase III trials conducted in the United States.
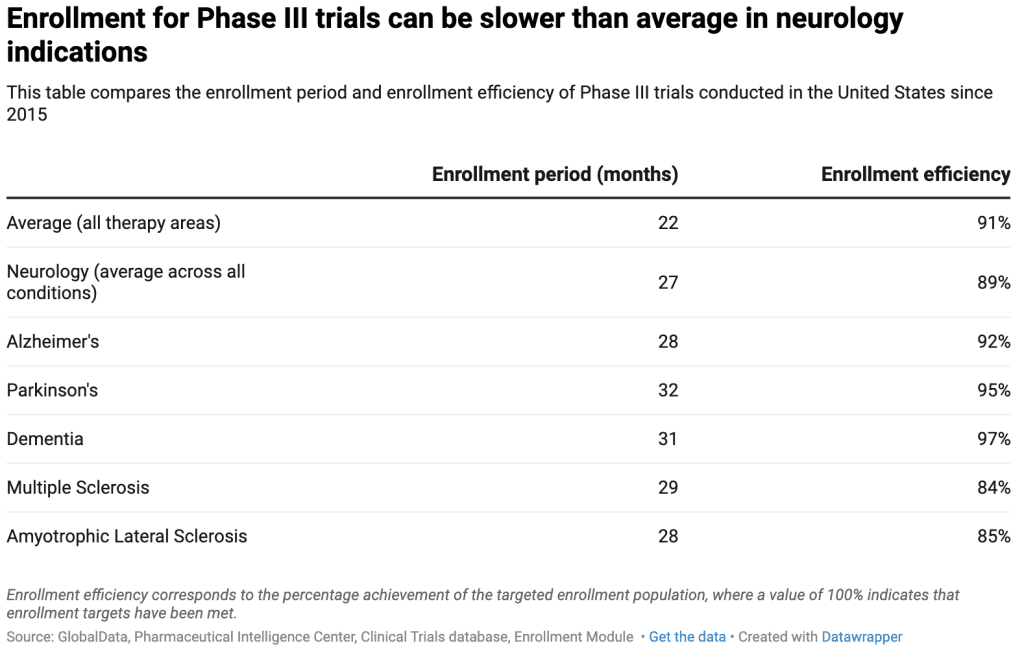
Overall, the data shows that Phase III neurology trials take four months longer to complete their enrollment period compared to trials in other therapeutic areas. Enrollment timelines can be even longer for certain neurodegenerative conditions, such as Parkinson’s disease and dementia. Other conditions also notably experience challenges with enrollment efficiency. Phase III trials for multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS), for example, show enrollment efficiency rates that are 6–7% below average.
Multiple factors contribute to the enrollment challenges highlighted above, including delayed diagnoses. Because the symptoms of neurodegenerative conditions are often mistaken by patients and their families as normal signs of aging, many individuals are not diagnosed until their disease has progressed too far to qualify for most clinical trials, which typically aim to test disease-modifying treatments before significant cognitive decline occurs. Additionally, older adults are more likely to have comorbidities that may disqualify them from participation.
Even when patients are eligible to participate, several aspects of the trial experience can pose additional barriers to recruitment. For example, traveling to a research site for study visits may be physically or logistically challenging for individuals with limited mobility, such as those recovering from a stroke or living with Parkinson’s disease. Similarly, the emotional and psychological stress of navigating unfamiliar and uncomfortable environments can be a significant deterrent for patients with dementia.
The study partner effect
In trials for late-stage neurodegenerative diseases, participants may be required to have a study partner—typically a family member or caregiver—who can accompany them to site visits, provide information, help manage distress, and support adherence to the study protocol. While study partners play a vital role in the success of neurology research, their involvement means that sponsors must effectively recruit and engage not only the participants themselves, but also their support networks. The role of the study partner becomes even more pivotal when considering how different types of partners can influence enrollment and retention. In 2012, researchers at UCLA analyzed over 2,000 participants across six Alzheimer’s trials and discovered that, although approximately 90% of Alzheimer’s patients in the general population do not have spouses, 67% of trial participants had a spouse as their study partner. This means that a significant portion of the patient population – those who may rely on adult children or other caregivers for support – is being underrepresented in clinical research.
The study also found that the participants with “non-spousal” study partners, such as adult children, were more likely to come from minority backgrounds. Specifically, they were twice as likely to be Hispanic and nearly three times as likely to be African American. Notably, dropout rates were 70% higher among participants with non-spousal study partners compared to those accompanied by their spouse. This disparity is likely driven by logistical challenges: non-spousal partners are less likely to live with the participant and more likely to have competing family or work responsibilities, making consistent trial participation more difficult.
Why site-centric visit models fall short in neurology trials
The challenges of participation are exacerbated by the demanding nature of the traditional site-centric visit model, which often requires participants to travel for frequent visits to the study site, many of which are commonly located in large cities and urban centers. Given the long treatment durations required to assess disease progression over time, retaining participants becomes increasingly challenging as trials progress.
Moreover, the geographic distribution of clinical research sites across the United States is uneven. An analysis of ongoing neurology trials shows that California, Florida, and Ohio are the most active states for recruitment, while Boston, New York, Las Vegas, Los Angeles, and Houston are the leading cities. Many leading research sites are currently recruiting for more than ten neurology trials, such as Massachusetts General Hospital in Boston, which is enrolling participants in 15 active studies.
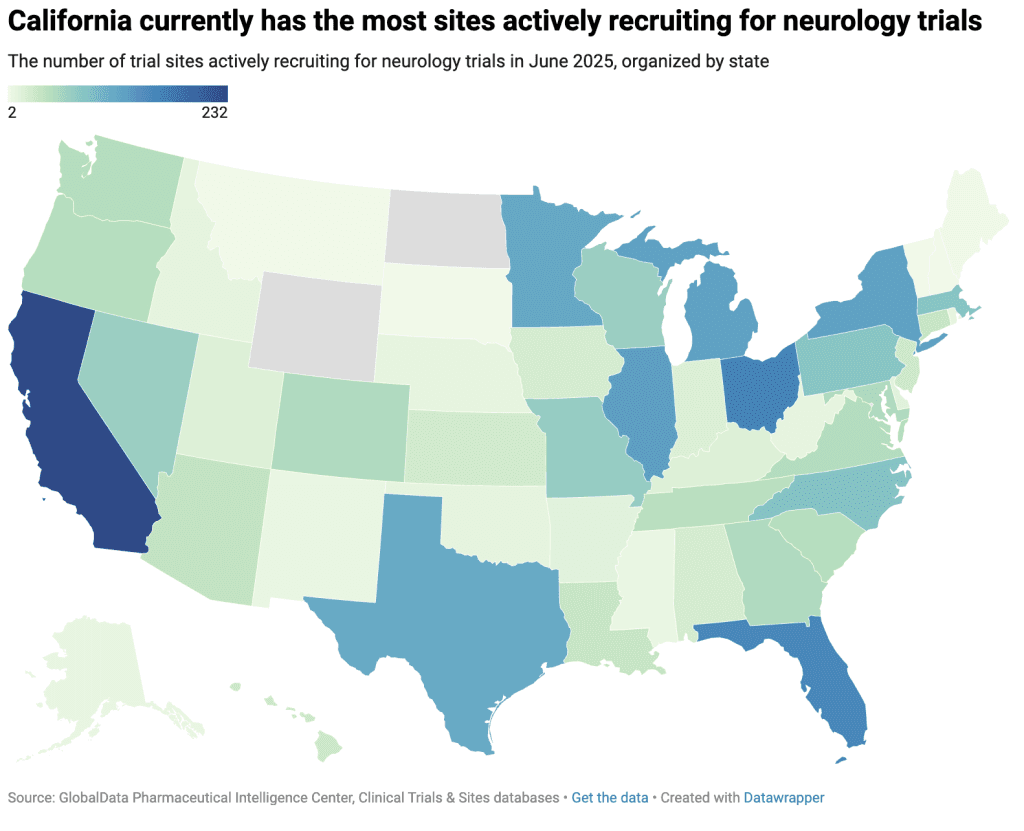
This concentration of research activity means that neurology sponsors may face intense competition to recruit from the same limited patient pools. Meanwhile, rural or research-naïve communities are often overlooked, representing a missed opportunity and contributing to the lack of access for patients living outside established neurology research hotspots.
This imbalance becomes even more apparent when looking at the distribution of neurology trial sites in certain states. In Utah, 89% of actively recruiting neurology trials are based in Salt Lake City, even though just 6% of the state’s population lives in the city itself, and just 35% reside within its metropolitan statistical area (MSA). Similarly, in Alabama, 63% of neurology trials are recruiting from sites in Birmingham, while only 4% of the state’s population lives in the city and 23% within the Birmingham MSA.
Community-based trials could help
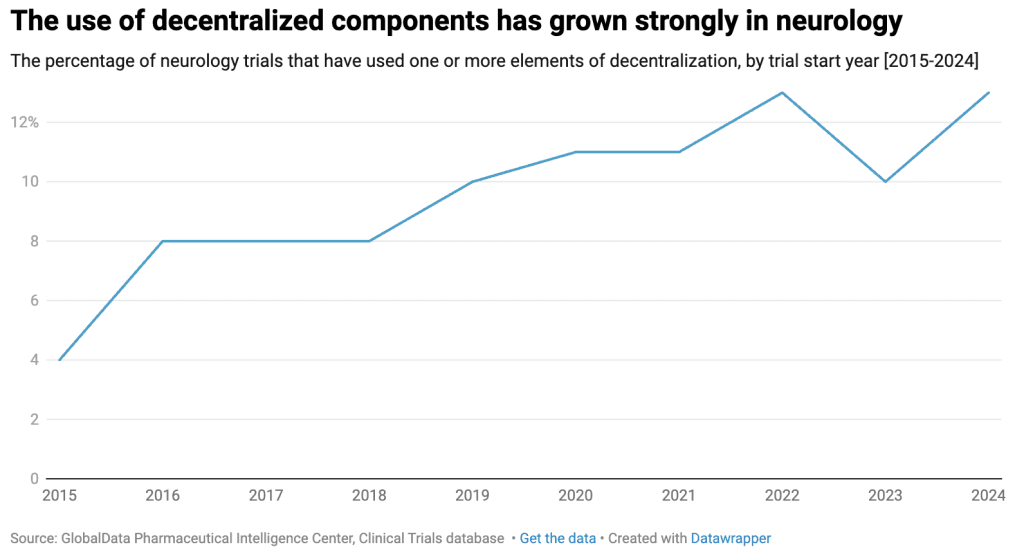
Where brick-and-mortar infrastructure may be lacking, community-based research approaches offer a valuable alternative. While the COVID-19 pandemic is often credited with accelerating the shift toward decentralized and community-based clinical research, the adoption of these methods in neurology trials had already been gaining momentum well before the pandemic.
PCM Trials is one company that has played a first-hand role in advancing the adoption of community-based research solutions. Since 2008, PCM Trials’ network of expert mobile clinicians has supported clinical trials across a wide range of therapeutic areas by traveling directly to participants’ homes or other convenient locations to conduct study visits. More recently, PCM Trials expanded its capabilities by acquiring EmVenio Research, a company specializing in the development and operation of community-based research sites designed to expand access to research to improve enrollment and retention, particularly among underrepresented populations. In recent years, several examples have demonstrated the benefits of a community-based approach in neurology trials. In one case study, PCM Trials’ supported one of the largest randomized, placebo-controlled clinical trials ever attempted in Parkinsonism. As part of the trial’s fully home-based protocol, Certified Mobile Research Nurses traveled to participants’ homes to administer a one-time intravenous infusion, supporting increased convenience for patients with limited mobility and expanding the potential participant pool beyond what would have been possible with traditional site-based visits.
In another study, PCM Trials’ mobile clinicians performed monthly intravenous infusions in a Phase III Alzheimer’s trial. While mobile visits were originally introduced as a result of the COVID-19 pandemic, the 4.5-year-long study ultimately implemented remote infusions as a permanent option for participants, recognizing their potential to improve convenience, increase enrollment, boost retention, and enhance protocol adherence.
Mobile research units have also been deployed by PCM Trials to support screening efforts in a trial that sought to recruit pre-clinical Alzheimer’s patients. Given the trial’s high screen-failure rate, these units played a critical role in enabling the recruitment and screening of large numbers of potential participants, facilitating access to the study at the community level.
To learn more about the role of community-based research in advancing clinical trials, please download the whitepaper below.